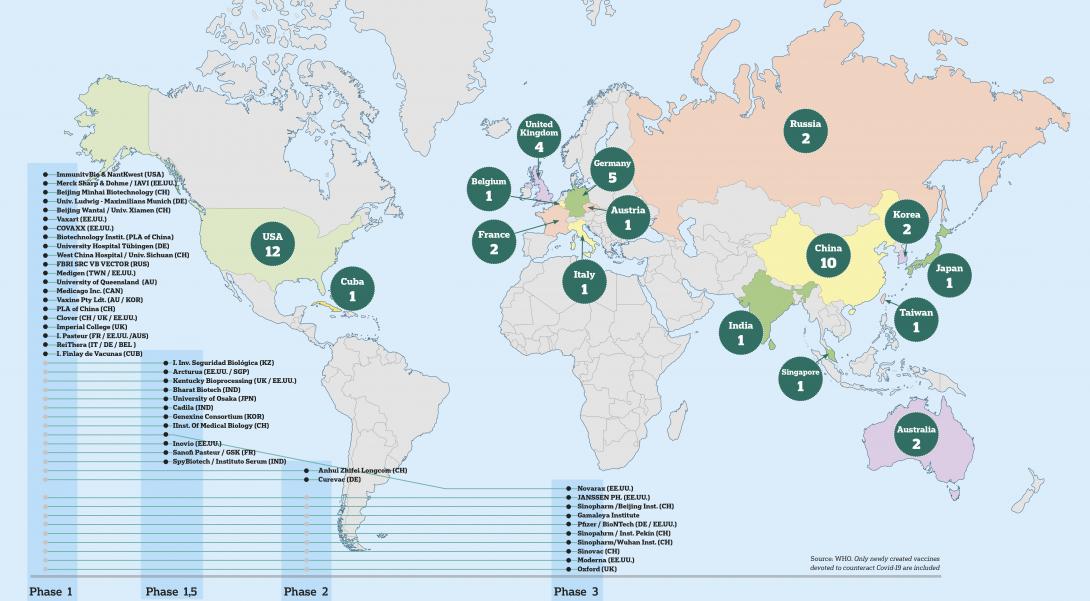
Worldwide prominent vaccine projects
The USA, China, Germany and the United Kingdom are leading the research to find a vaccine in as short a time as possible. Ten projects are already in phase three, which means that they are being tested on thousands of people. The creation process of a vaccine can take years, but international cooperation and the efforts of thousands of scientists are beginning to show results. The goal is to find a vaccine for the end of this year, or the beginning of 2021.
Since the World Health Organisation declared the outbreak of Covid-19 to be a pandemic, many pharmaceutical laboratories and public institutions have been working to obtain a vaccine. According to the latest data on the 15th of October, provided by the WHO, there are 151 projects in a pre-clinical phase and 10 have already reached phase 3, which is the step prior to marketing.
1 Sinovac
Country: China
Participants: Butantan Institute
Pharmaceutical company: Sinovac Life Sciences Co., Ltd.
The chairman of the Chinese pharmaceutical company Sinovac, Yin Weidong, affirmed at the end of September that their vaccine against the coronavirus could start being applied to the population en masse at the beginning of next year. Trials are being carried out on thousands of Brazilian volunteers after the country’s government gave the go ahead. It is also being tested in Turkey, Bangladesh and Indonesia.
2 and 3 Sinopharm
Country: China
Participants: Wuhan Institute of Biological Products and Beijing Institute of Biological Products.
Pharmaceutical company: Sinopharm
These are two independent projects that the same Chinese pharmaceutical company is researching, the State-run company, Sinopharm. Both are based on inactivated viruses. Regarding the project by the Beijing Institute of Biological Products and Sinopharm, in the middle of October the Chinese laboratory published an article in The Lancet with promising results, after carrying out a trial in Beijing with 1,192 people.
4 AstraZeneca
Country: United Kingdom
Participants: Jenner Institute of the University of Oxford
Pharmaceutical company: AstraZeneca
This is the most important project being carried out in Europe. It is using an attenuated version of the common cold virus in Chimpanzees. Phase 3 is being developed in Brazil, South Africa and the United Kingdom. On the 6th of September they had to suspend the trials because two of the volunteers became ill. A few days later, the Health Medicine Regulation Authority gave the go ahead for the trial to be restarted.
5 CanSino Biological Inc.
Country: China
Participants: Beijing Biotechnological Institute
Pharmaceutical company: CanSino Biological Inc.
Called Ad5-nCOV. It was the first vaccine candidate to be patented by the Chinese National Administration for Intellectual Copyright, meaning that it could be mass produced in the near future. Trials were started in June with members of the Chinese army and the results of the second phase showed that it is safe and it induces an immune response against the coronavirus, as was published in The Lancet in July.
6 Gamaleya Research Institute
Country: Russia
Participants: Russian Ministry of Defence
Pharmaceutical company: —
In the middle of August, the President of Russia, Vladimir Putin, announced that his country was the first in the world to register a vaccine against the new coronavirus. The Russian Government authorised its administration. After the first results, the vaccine, called Sputnik V produced an antibody response in all the participants in the first stages of the clinical trials, according to The Lancet.
7 Janssen Pharmaceutical Companies
Country: United States
Participants: Johnson&Johnson
Pharmaceutical company: Janssen Pharmaceutical Companies
They have created the recombinant vaccine Ad26.COV2-S, based on an adenovirus that can generate an immune response against the S protein of the coronavirus. Phase 3 has been started with 60,000 volunteers in the USA. It could be on the market at the beginning of 2021. Janssen signed agreements with Emergent BioSolutions, Inc and Catalent biologics to support the manufacturing of a billion doses.
8 Novavax
Country: United States
Participants: Zendal
Pharmaceutical company: Novavax
The biotechnology company has developed the vaccine against Covid-19, NVCXCoV2373. The first results from the clinical trials have shown that it is safe and causes an immune response, according to a study published at the beginning of September in The New England Journal of Medicine. The pharmaceutical company has the capacity to manufacture 2,000 million doses for mid-2021.
9 Moderna
Country: United States
Participants: Biomedical Advanced Research and Development Authority and National Institute of Allergy and Infectious Diseases (NIAID)
Pharmaceutical company: Moderna
The vaccine named mRNA-1273 is based on the messenger RNA combined with the virus’ genetic code. It has the European Medicines Agency’s confirmation to request a marketing authorisation by the EU. It is in phase 3 and the 22,194 participants in the study received the second dose in October.
10 BioNTech/Fosun Pharma/Pfizer
Country: Germany and United States
Pharmaceutical company: BioNTech, Fosun Pharma y Pfizer
It has four variants based on the synthetic messenger RNA. In July, Pfizer and BioNtech showed that one of their vaccine trials against Covid-19 was safe and generated antibodies against the new coronavirus strain in patients. In October, Pfizer announced that it would request an emergency authorisation for the use of its vaccine against Covid-19 at the end of November.